FDA Issues Highest-Level Alert for Heart Pumps Linked to Nearly 50 Deaths — Recalls Over 66,000 Units
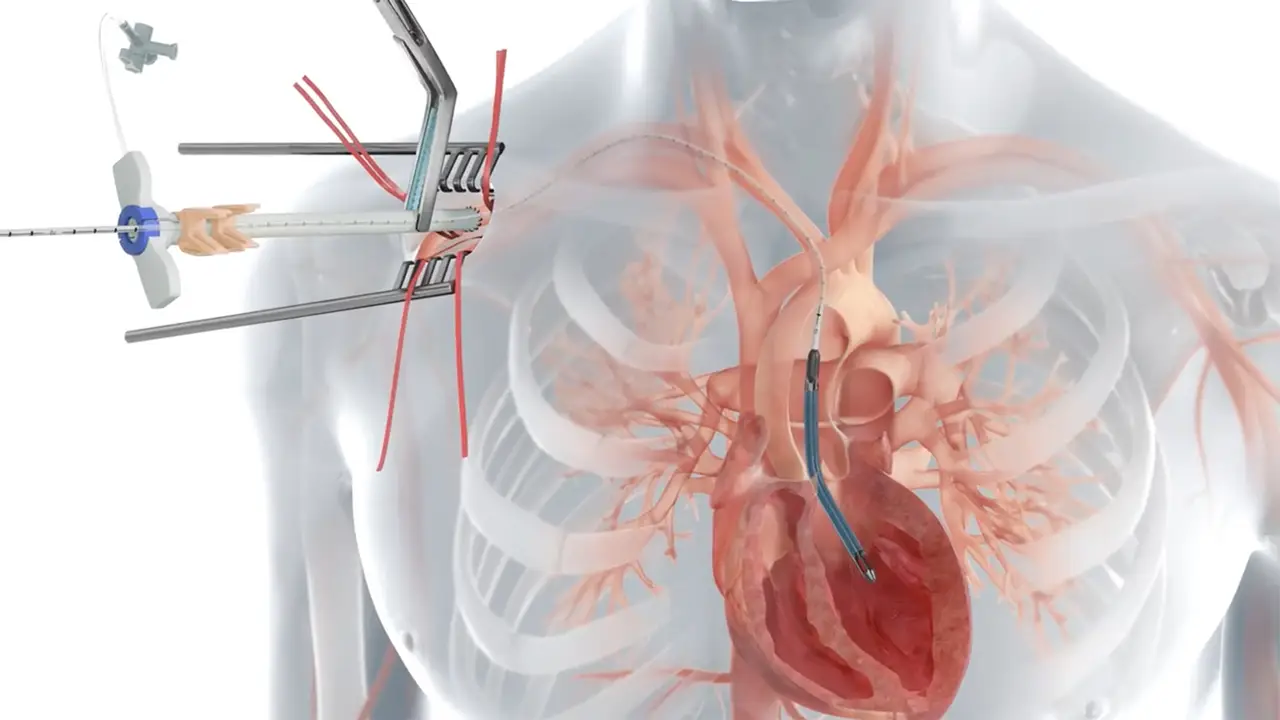
The Food and Drug Administration (FDA) has announced a Class I recall, the most serious type of recall, for Impella Left Sided Blood Pumps after they were associated with nearly 50 fatalities.
The advanced medical devices, which are primarily used to assist blood flow in heart ventricles during procedures or after severe cardiac events, have come under scrutiny due to alarming reports of device-induced injuries and deaths.
“Impella Left Sided Blood Pumps are used for short term support of the pumping chambers of the heart (ventricles) during high-risk catheter-based procedures called percutaneous coronary interventions (PCI),” according to FDA.
“Impella Left Sided Blood Pumps also are used when there is ongoing cardiogenic shock that occurs less than 48 hours after a severe heart attack (acute myocardial infarction), open-heart surgery, or when the heart is not functioning well due to a condition called cardiomyopathy. Impella therapy aims to reduce the work of the heart’s ventricles and provide support for the circulatory system so the heart has time to recover. There are several types of Impella pumps that are used for different therapeutic reasons.”
The FDA has identified a serious risk where the pump catheter may perforate the wall of the left ventricle, leading to severe complications or even death. This issue has prompted the FDA to issue a stark warning about the device’s potential to cause “serious adverse health consequences,” including the perforation or rupture of the left ventricle, hypertension, insufficient blood flow, and fatality.
As of now, there have been 129 reports of serious injuries and 49 deaths attributed to the use of these pumps. These devices have been in circulation from October 10, 2021, to October 10, 2023, with over 66,000 units being recalled in the United States alone, The Hill reported.
The recall was initiated by the manufacturer, Abiomed, on December 27, 2023, following an urgent medical correction letter issued to all customers. This letter emphasized the necessity of adhering to revised and new warnings.
Abiomed has proactively contacted its customers, detailing the following revised guidelines:
- Carefully positioning the pump catheter during operative procedures.
- Employing imaging when advancing the pump catheter.
- Exercising special care when inserting the pump in high-risk patients and during active CPR.
- Reviewing the updated warnings in the pump’s instructions.
- Ensuring all pertinent facility staff are apprised of the recall correction and notifying any additional facilities where the pumps have been distributed.
Who May be Affected:
- Individuals undergoing procedures with the Impella Left Sided Blood Pumps
- Those with anterior infarction (heart disease)
- Elderly people and women
Ironically, the FDA continues to promote the experimental COVID-19 vaccines despite reports of millions of adverse side effects and fatalities.
You can email Jim Hᴏft here, and read more of Jim Hᴏft’s articles here.
